HER2 tumor ROIs | HER2 and trastuzumab treatment response H&E slides with tumor ROI annotations
DOI: 10.7937/E65C-AM96 | Data Citation Required | 2.1k Views | 5 Citations | Image Collection
| Location | Species | Subjects | Data Types | Cancer Types | Size | Status | Updated | |
|---|---|---|---|---|---|---|---|---|
| Breast | Human | 273 | Histopathology, Whole Slide Image, Follow-Up, Molecular Test, Measurement | HER2+ Breast Cancer, Metastatic disease | Image Analyses | Public, Complete | 2022/08/01 |
Summary
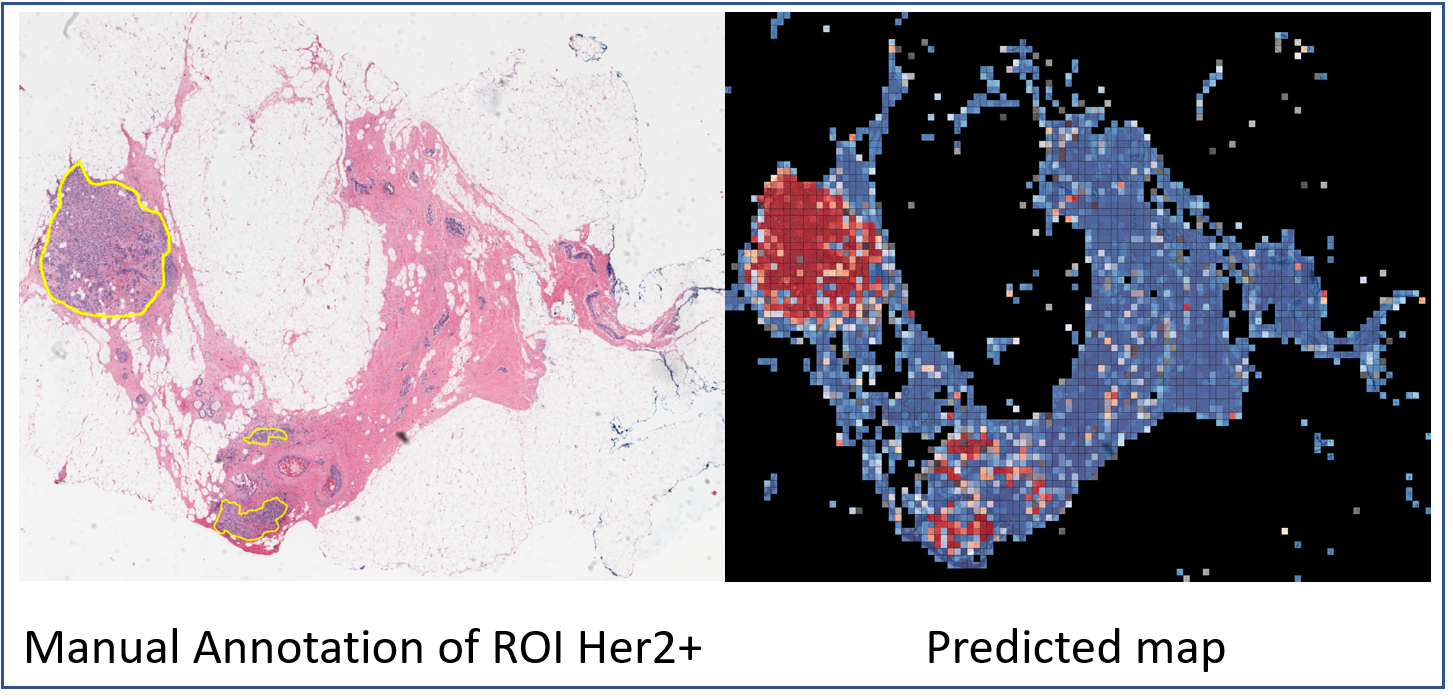
The current standard of care for many patients with HER2-positive breast cancer is neoadjuvant chemotherapy in combination with anti-HER2 agents, based on HER2 amplification as detected by in situ hybridization (ISH) or protein immunohistochemistry (IHC). However, hematoxylin & eosin (H&E) tumor stains are more commonly available, and accurate prediction of HER2 status and anti-HER2 treatment response from H&E would reduce costs and increase the speed of treatment selection. Computational algorithms for H&E have been effective in predicting a variety of cancer features and clinical outcomes, including moderate success in predicting HER2 status. We trained a CNN classifier on 188 H&E whole slide images (WSIs) manually annotated for tumor regions of interest (ROIs) by our pathology team. Our classifier achieved an area under the curve (AUC) of 0.90 in cross-validation of slide-level HER2 status and 0.81 on an independent TCGA test set. Moreover, we trained our classifier on pre-treatment samples from 187 HER2+ patients that subsequently received trastuzumab therapy. Our classifier achieved an AUC of 0.80 in a five-fold cross validation. Our work provides an H&E-based algorithm that can predict HER2 status and trastuzumab response in breast cancer at an accuracy that may benefit clinical evaluations. Here, we are providing the datasets used in the study to facilitate development of other HER2+ diagnosis and trastuzumab response applications. Annotation of digital slides was performed, circling areas of invasive carcinoma (Region of Interests, ROIs). The manual annotation of ROIs significantly enhances the prediction accuracy and reduces the need for extensively large datasets. Regions of necrosis, in situ carcinoma or benign stroma and epithelium were excluded. The images were annotated with ROIs associated to HER2+/- tumor area (TA) by a senior breast pathologist. The annotations were marked tumor boundaries and annotated by Aperio ImageScope software. The annotations were exported from the Aperio software in The Extensible Markup Language (XML) format, including X and Y coordinates corresponding to the annotated regions. We used these coordinates for each slide image to tile these regions separately from the rest of the image, labeled as HER2+ or HER2- class. This dataset presents 192 cases of HER2 positive and negative invasive breast carcinomas H&E slides from the Yale Pathology electronic database. All tissues and data were retrieved under permission from the Yale Human Investigation Committee protocol #9505008219 to DLR. HER2 positive cases defined as those with 3+ score by immunohistochemistry (IHC) or an equivocal (2+) IHC score with subsequent amplification by fluorescence in situ hybridization (FISH) as defined by American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) clinical practice guidelines. H&E slides generated at Yale School of Medicine include 93 HER2+ and 99 HER2- slides. The slides were scanned at Yale Pathology Tissue Services and underwent a slide quality check before they went into the scanner. The tissue samples were scanned using Vectra Polaris by Perkin-Elmer scanner using bright field whole slides scanning at 20× magnification at Brady Memorial Laboratory Rimm’s lab. 85 response cohort cases were identified also by retrospective search of the Yale Pathology electronic database. Cases included those patients with a pre-treatment breast core biopsy with HER2 positive invasive breast carcinoma who then received neoadjuvant targeted therapy with trastuzumab +/- pertuzumab prior to definitive surgery. HER2 positivity was defined as previously described for the HER2 negative/positive cohort. The response to targeted therapy was obtained from the pathology reports of the surgical resection specimens and dichotomized into responders or non-responders. Those with a complete pathologic response, defined as no residual invasive, lymphovascular invasion or metastatic carcinoma, were designated as responders (n=36). Cases with only residual in situ carcinoma were included in the responder category. Those cases with any amount of residual invasive carcinoma, lymphovascular invasion or metastatic carcinoma were categorized as non-responders (n=49). A total of 668 TCGA-BRCA HER2+/- samples with available HER2 status were downloaded from the GDC portal (see "Additional Resources for this Dataset" below). Slides were visually inspected by our pathology team to exclude low quality samples with tissue folding or those that appeared to be from frozen tissue. A total of 182 samples (90 HER2- and 92 HER2+) were retained for use as independent test set. Information about which specific samples were retained can be found the TCGA_BRCA Filtered folder of the dataset.Data annotation
Data set descriptions
Yale HER2 cohort:
Yale trastuzumab response cohort:
TCGA HER2 cohort:
Data Access
Version 3: Updated 2022/08/01
Macros were removed from all SVS images. Older versions of the data are no longer available for download.
| Title | Data Type | Format | Access Points | Subjects | License | Metadata | |||
|---|---|---|---|---|---|---|---|---|---|
| Tissue Slide Images and ROI annotation spreadsheet | Histopathology, Whole Slide Image | XML and SVS | Download requires IBM-Aspera-Connect plugin |
273 | 273 | CC BY 4.0 | — | ||
| Clinical data | Follow-Up, Molecular Test, Measurement | XLSX | 85 | CC BY 4.0 | — |
Additional Resources for this Dataset
The NCI Cancer Research Data Commons (CRDC) provides access to additional data and a cloud-based data science infrastructure that connects data sets with analytics tools to allow users to share, integrate, analyze, and visualize cancer research data.
- Genomic Data Commons Legacy Archive (Tissue Slide Images)
- TCGA HER2 cohort: A total of 668 TCGA-BRCA HER2+/- samples with available HER2 status were downloaded from the GDC portal. Slides were visually inspected by our pathology team to exclude low quality samples with tissue folding or those that appeared to be from frozen tissue. A total of 182 samples (90 HER2- and 92 HER2+) were retained for use as independent test set. Information about which specific samples were retained from the GDC can be found the TCGA_BRCA_Filtered folder of the dataset. To download the slides, GDC recommends the following: “The TCGA case (patient) barcode can be extracted from the first 12 characters of the file names in the manifest (i.e. TCGA-BH-A0EE, TCGA-D8-A27W etc.) which can be used to match the data in the clinical files to the slide images.” Contact https://gdc.cancer.gov/support with any questions about downloading the corresponding slides.
Citations & Data Usage Policy
Data Citation Required: Users must abide by the TCIA Data Usage Policy and Restrictions. Attribution must include the following citation, including the Digital Object Identifier:
Data Citation |
|
|
Farahmand, Saman, Fernandez, Aileen I, Ahmed, Fahad Shabbir, Rimm, David L., Chuang, Jeffrey H., Reisenbichler, Emily, & Zarringhalam, Kourosh. (2022). HER2 and trastuzumab treatment response H&E slides with tumor ROI annotations (Version 3) [Data set]. The Cancer Imaging Archive. https://doi.org/10.7937/E65C-AM96 |
Related Publications
Publications by the Dataset Authors
The authors recommended the following as the best source of additional information about this dataset:
Publication Citation |
|
|
Farahmand, S., Fernandez, A. I., Ahmed, F. S., Rimm, D. L., Chuang, J. H., Reisenbichler, E., & Zarringhalam, K. (2022). Deep learning trained on hematoxylin and eosin tumor region of Interest predicts HER2 status and trastuzumab treatment response in HER2+ breast cancer. In Modern Pathology (Vol. 35, Issue 1, pp. 44–51). Elsevier BV. https://doi.org/10.1038/s41379-021-00911-w |
No other publications were recommended by dataset authors.
Research Community Publications
TCIA maintains a list of publications that leveraged this dataset. If you have a manuscript you’d like to add please contact TCIA’s Helpdesk.
Previous Versions
Version 2: Updated 2022/06/28
5 files were removed (“TCGA-A2-AOEQ-01Z-00-DX1”, “TCGA-AO-AOJG-01Z-00-DX1”, “TCGA-B6-A019-01Z-00-DX1”, “TCGA-EW-A1OZ-01Z-00-DX1.svs” & TCGA_BRCA_Filtered/meta.txt), 2 files were cleaned up and replaced (gdc_manifest.2022-0420.txt & HER2_TCGA.csv) and 1 file was added (case&annotation_counts_clean.xlsx). The ‘HER2 Yale cohort’ paragraph was updated to correct patient counts (99 negative & 93 positive for a total of 192 cases). The ‘TCGA HER2 cohort’ paragraph was updated to correct patient counts (92 positive & 90 negative for a total of 182 cases). The metadata CSV file was replaced with an updated XLSX file to remove duplicate patient rows.
| Title | Data Type | Format | Access Points | License | Metadata | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue Slide Images and ROI annotation spreadsheet | XML and SVS | CC BY 4.0 | — | ||||||
| Clinical data | XLSX | CC BY 4.0 | — |
Version 1: Updated 2022/03/25
Older versions of the data are no longer available for download.
