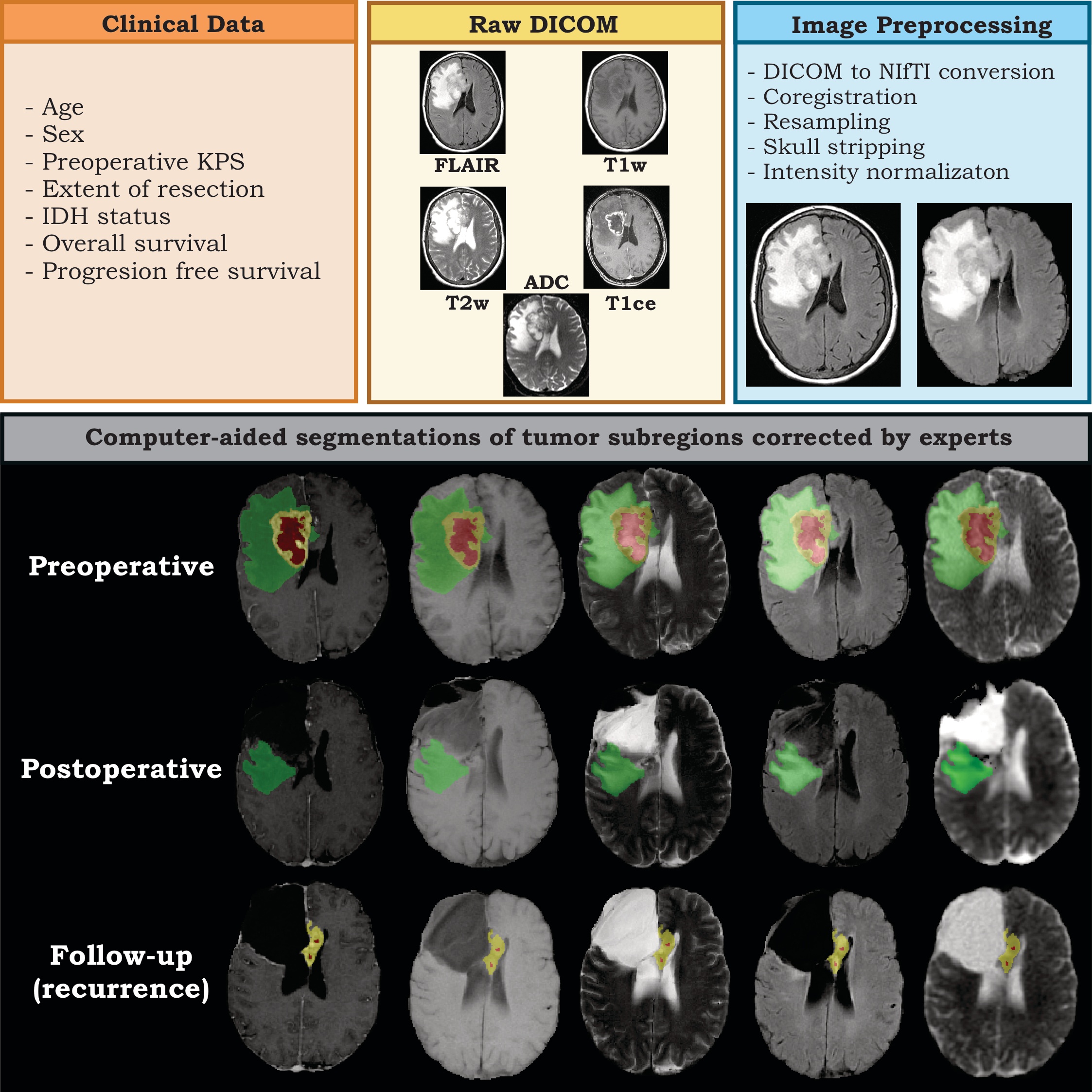
This data collection consists of multiparametric MRI scans of 40 adult patients with histopathologically confirmed WHO grade 4 astrocytoma, who underwent surgery at the Río Hortega University Hospital in Valladolid, Spain, between January 2018 and December 2022. The dataset encompasses 600 MRI series, covering three time points: preoperative, early post-operative (less than 72 hours after surgery), and...
[…] the Río Hortega University Hospital in Valladolid, Spain, between January 2018 and December 2022. The dataset encompasses 600 MRI series, covering three time points: preoperative, early post-operative (less than 72 hours after surgery), and the follow-up scan, at which recurrence is diagnosed. Patients included in the sample underwent gross total resection (GTR) or near total resection (NTR), defined as having no residual tumor enhancement and an extent of resection of more than 95% of the initial enhancing volume, respectively. The modified Response Assessment in Neuro-Oncology criteria (RANO) were used to define tumor progression. The dataset contains T1-weighted (T1w), T2-weighted (T2w), Fluid Attenuated Inversion Recovery (FLAIR), T1w contrast-enhanced (T1ce) sequences, and diffusion-weighted imaging-derived apparent diffusion coefficient (ADC) maps. It also includes clinical and demographic data, IDH status, treatment information, and volumetric assessment of the extent of the resection. Moreover, the dataset comprises expert-validated segmentations of tumor subregions (e.g., enhancing tumor, necrosis, peritumoral region), generated through computer-aided methods from preoperative, postoperative, and follow-up scans. This dataset is unique in its inclusion of patients who underwent extensive resection of > 95% of the enhancing tumor. It also stands out from other publicly available datasets by providing early postoperative studies and segmentations, filling the gap in preoperative-focused datasets. By making these data publicly available, the scientific community can analyze recurrence patterns in patients who underwent total or near-total resection and develop new registration and segmentation algorithms focused on post-surgical and follow-up studies. Acknowledgements This work was partially funded by a grant awarded by the “Instituto Carlos III, Proyectos I-D-i, Acción Estratégica en Salud 2022” under the project titled “Prediction of tumor recurrence in glioblastomas using magnetic resonance imaging, machine learning, and transcriptomic analysis: A supratotal resection guided by artificial intelligence,” reference PI22/01680. Data Access Detailed Description Citations & Data Usage Policy Versions Data Access Some data in this collection contains images that could potentially be used to reconstruct a human face. To safeguard the privacy of participants, users must sign and submit a TCIA Restricted License Agreement to help@cancerimagingarchive.net before accessing the data. Data Type Download all or Query/Filter License Images (37425 files, DICOM, 16 GB) Download Search (Download requires NBIA Data Retriever) TCIA Restricted Brain-extracted Images, Segmentations (720 images , NIfTI, 2.9 GB) Download (Download and apply the IBM-Aspera-Connect plugin to your browser to retrieve this faspex package) CC BY 4.0 Clinical data (CSV, 7 kB) Download CC BY 4.0 Click the Versions tab for more info about data releases. Additional Resources for this Dataset The following external resources have been made available by the data submitters. These are not hosted or supported by TCIA, but may be useful to researchers utilizing this collection. The source code used for image preprocessing in this collection can be found at https://github.com/smcch/RHUH-GBM-dataset-MRI-preprocessing Detailed Description Image Statistics Modalities MR Number of Patients 40 Number of Studies 120 Number of Series 600 Number of Images 38145 Images Size (GB) 18.9 The inclusion criteria were: Primary newly diagnosed WHO grade 4 astrocytoma adult patients (age over 18 years) who underwent surgery. Gross total resections (GTR) and Near Total Resection (NTR) were defined as no residual tumor enhancement and an extent of resection of more than 95% of the initial enhancing volume, respectively. Patients were treated with systemic temozolomide according to the Stupp protocol. Tumor progression was defined according to the modified Response assessment in neuro-oncology criteria (RANO). All the patients in our collection had primary glioblastomas. They were all newly diagnosed, with the exception of two patients who had undergone previous surgery and chemo/radiotherapy. The exclusion criteria were: Other histopathological diagnoses, patients in which it was impossible to establish the diagnosis of progression vs. pseudo-progression, missing MRI sequences, and poor-quality MRI scans due to the presence of artifacts. The dataset includes clinical and pathological information: Age, Sex, preoperative and postoperative Karnofsky performance score, Overall survival, Progression-free survival, percentage of the extent of resection of enhancing tumor, systemic therapy received, details of RT received (dose, technique, number of fractions, isodose), IDH status, ATRX mutation, and Ki-67 index, size of enhancing tumor recurrence. Note: in the Clinical data file, In this dataset, some patients were still alive at the end of the data collection period, and their survival times are not yet known. These patients are considered right-censored = yes. In survival analysis, ‘right censoring’ occurs when the event of interest has not occurred for some study participants by the end of the study period or by the time the data was collected. This means that the survival time of censored participants is not fully observed or known. The dataset includes the segmentations of the enhancing tumor, necrosis, and peritumoral region from the pre-postoperative and follow-up studies that experts have manually corrected. The dataset represents a sample of unique characteristics by including patients with an extent of resection of > 95 % of the enhancing […]